The sports journalist David Epstein uses Rogers’ experience as his opening example of the significant, underappreciated benefits of delaying specialization and accumulating a breadth of different experiences.
For most people, the highly effective mRNA COVID vaccines made by Moderna and Pfizer-BioNTech seemed to come out of the blue. But these new vaccines, which were essential to end this pandemic and will likely play a critical role in preventing future pandemics, are the product of decades of painstaking work by researchers.
One of those researchers is Dr. Katalin Karikó, a Hungarian biochemist who long ago saw the potential of mRNA to save lives when few others did.
The daughter of a small-town butcher in Hungary, Karikó knew from a young age that she wanted to become a scientist. She was drawn to biochemistry and developed a particular fascination with messenger RNA, or mRNA, molecules that (among other things) direct the creation of proteins in your body.
Messenger RNA functions as a kind of middleman—it carries the directions for making proteins from your DNA to the factories in your cells where the proteins will be assembled. It’s a bit like the waiter in a restaurant who writes down your order and takes it to the kitchen, where the cooks will make your meal.
In the 1980s, while working on her PhD in her native Hungary, Karikó became convinced that tiny strands of mRNA could be injected into cells to send instructions to the body to make its own medicines. She was interested in developing mRNA treatments for stroke, cancer, and other diseases.
Although vaccines were not the focus of Karikó’s work, other researchers saw that it would be possible to use mRNA to make those as well—for flu, coronaviruses, and maybe even various forms of cancer.
Using mRNA to make vaccines would be a major departure from the way most vaccines work. Many conventional vaccines operate by injecting a weakened or dead form of the virus you’re trying to stop. Your immune system sees the new shapes on the virus, kicks into gear, and builds up immunity. While conventional vaccines have been very effective, it takes years of lab work and clinical studies to make sure that they are safe and will produce a good immune response.
The idea behind mRNA vaccines was quite clever. Since mRNA takes the orders for proteins from the DNA and delivers them to the cooks in your cells’ kitchen, what if we could change those orders in a very targeted way? By teaching your cells to make shapes that match shapes on the actual virus, the vaccine would trigger your immune system without having to introduce the virus itself.
If they could be made, mRNA vaccines would be a huge advance over conventional vaccines. Once you had mapped out all the proteins that make up the virus you wanted to target, you’d identify the one that you want antibodies to grab. Then you’d study the virus’s genetic code to find the instructions for making that protein, and you’d put that code into the vaccine using mRNA. If, later, you wanted to attack a different protein, you’d just change the mRNA. This design process would take at most a few weeks. You would ask the waiter for fries instead of a side salad, and your immune system would do the rest.
There was just one problem: It was only a theory. No one had ever actually made an mRNA vaccine. What’s more, most people in the field thought it was crazy to even try, not least because mRNA is inherently unstable and prone to degrading quickly. Also, cells have evolved to avoid being hijacked by foreign mRNA, and there would need to be a way of getting around this defense system.
Karikó’s interest in mRNA eventually brought her to the U.S. And in 1993, while doing research at the University of Pennsylvania, Karikó and her boss managed a feat that told them they were on to something: They got a human cell to produce a tiny amount of new proteins using a modified version of mRNA that had been altered so it could get past the cell’s defense system. This was a breakthrough, because it meant that if they could expand the production dramatically, they would be able to make a cancer treatment using mRNA.

Dr. Katalin Karikó, who knew from a young age that she wanted to become a scientist, works in her lab, 1985. (personal photo/Katalin Karikó)
Stories of medical discoveries often don’t travel in straight line from breakthrough to lifesaving impact. And Karikó’s story is no different. Karikó’s work lost momentum when her boss left academia for a biotech firm. She no longer had a lab or financial support for her work; although she applied for grant after grant, every application was rejected. In 1995, she had a cancer scare, she was taken off the tenure track at work, and her husband was stuck in Hungary because of a problem with his visa. But Karikó was undeterred.
Then in 1997, she began working with Drew Weissman, a new colleague who came to the University of Pennsylvania with a promising background: He had done a fellowship at NIH under the supervision of Tony Fauci, and he was interested in using Karikó’s work on mRNA to develop vaccines.
Together Karikó and Weissman kept pursuing the idea of working with mRNA that had been engineered in a lab. But they still had to get more mRNA past the cell’s defense systems, a problem that other scientists helped solve. In 1999, a cancer researcher named Pieter Cullis and his colleagues proposed that lipids—basically, tiny bits of fat—could be used to encase and protect a more delicate molecule, such as mRNA. Six years later, working with Cullis, biochemist Ian MacLachlan did it for the first time. The lipid nanoparticles he developed paved the way for the first mRNA vaccines.

At their University of Pennsylvania lab, Dr. Katalin Karikó and her colleague Dr. Drew Weissman helped develop the technology that made mRNA vaccines possible.(University of Pennsylvania Medical School)
As late as 2010, hardly anyone in the federal government or private industry was interested in trying to make vaccines using mRNA. Major pharmaceutical companies had tried and failed, and some scientists felt that mRNA would never trigger enough of a response in the body. But an official at DARPA, the little-known research program for the U.S. military, saw enough promise in the technology that he started funding mRNA vaccines for infectious diseases.
As pioneering as this work was, it didn’t lead immediately to new vaccines. Accomplishing that would be the task of companies dedicated to translating the breakthrough into a product that could be approved and sold; the U.S.-based Moderna and Germany-based CureVac and BioNTech were founded to do just that.
In 2014, Karikó joined BioNTech, which was working on an mRNA vaccine for cancer. Early efforts didn’t work, although a test of a rabies vaccine showed promise. Still, Karikó and her BioNTech colleagues persevered, as did scientists at Moderna. When COVID hit, they immediately set out to make a vaccine for the new virus. It was a good bet.
The notion that mapping a virus’s genome would allow you to create an mRNA vaccine in a matter of weeks proved to be exactly right. In March 2020, just six weeks after scientists sequenced the COVID virus’s genome, Moderna announced that it had identified an mRNA-based candidate and begun making it for clinical trials. On December 31, the mRNA vaccine made by BioNTech in partnership with Pfizer was approved for emergency use by the World Health Organization. When Karikó received the first dose of the vaccine she had done so much to create—a few days before it was officially approved—she wept.
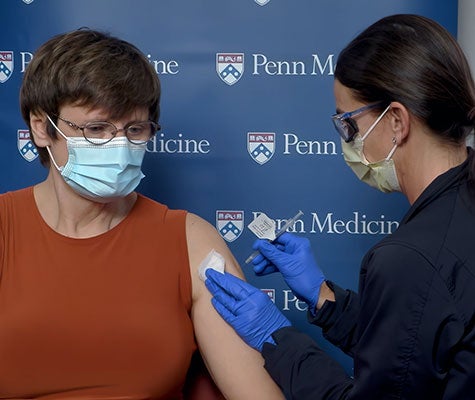
When Dr. Katalin Karikó got her first dose of the vaccine she had done so much to create, she wept. (University of Pennsylvania Medical School)

Dr. Katalin Karikó, Senior Vice President of BioNTech, celebrates with the co-founders of BioNTech after being presented with the German Future Prize award in Berlin, Germany, 2021 for their work on the COVID-19 vaccine. (Picture Aliiance/Getty Images)
When Dr. Katalin Karikó got her first dose of the vaccine she had done so much to create, she wept. (University of Pennsylvania Medical School)
Dr. Katalin Karikó, Senior Vice President of BioNTech, celebrates with the co-founders of BioNTech after being presented with the German Future Prize award in Berlin, Germany, 2021 for their work on the COVID-19 vaccine. (Picture Aliiance/Getty Images)
For all her amazing foresight, I doubt even Dr. Karikó imagined that mRNA vaccines would one day play an essential role in ending a pandemic – and giving us a tool to prevent the next one. And to me, that’s the important lesson of her story: It’s impossible to predict exactly how breakthroughs will shape the future. That’s why it’s critical, if the science makes sense, that we should be willing to bet on crazy sounding ideas and the researchers like Dr. Kariko willing to fight tooth and nail to pursue them. They just might change the world.
Meet more of my heroes in the field